BioHale® Sucrose
BioHale® Sucrose
High purity, low endotoxin sucrose
- CAS Number: 57-50-1
With BioHale® Sucrose we offer the highest purity excipient for the stabilization of biologics to be used in biopharmaceutical formulation.
Low stock: 8 left
Product description
Product description
BioHale® Sucrose is a non-reducing crystalline disaccharide made up of one glucose and one fructose molecule joined via a glycosidic linkage. Commonly used in the pharmaceutical industry as a stabilizer, Sucrose can be also widely used as a cryopreservative and media supplement in a variety of cell-based bioprocesses. Additionally, BioHale® Sucrose is preferred in applications where solubility or viscosity issues are of supreme importance.
BioHale® Sucrose is well suited to provide solution-state stabilization, as well as cryo- and lyo-protection for therapeutic proteins as excipients in the formulations. Sucrose can generally be considered as suitable in most cases, unless constrained by a low-pH formulation. BioHale® Sucrose can be used in various administration forms, such as for parenteral, oral or ophthalmic route.
Benefits
Benefits
Stabilizing agent
BioHale® Sucrose is a non-reducing sugar and does not react with amino acids or proteins, inhibiting the Maillard reaction. BioHale® Sucrose provides solution-state stabilization to fragile biomolecules.
Cryo- and lyoprotectant
BioHale® Sucrose, a high purity disaccharide excipient, protects the biologic drugs from the freeze related (cryoprotectant) and drying related (lyoprotectant) stresses. This makes BioHale® Sucrose particularly suitable in the stabilization process of today’s biologics.
Typical product data
Typical product data
• Description: White, or almost white, crystalline
powder, or lustrous, colorless or white, or
almost white, crystals
• Source: Plant derived, isolated from sugar beet
• Molecular Formula: C12H22O11
• Molecular Weight: 342.30 g/mol
• CAS Number: 57-50-1
Product specifications
Product specifications
Endotoxin: ≤ 0.25 EU/g
Heavy Metals: ≤ 5 ppm
Elemental Impurities: Complies with ICH Q3D
Total Impurities: ≤ 2.0%
Reducing sugars: ≤ 0.07
Quality
Quality
• High purity, low endotoxin grade produced by
active purification process
• Manufactured in The Netherlands, Europe
• FDA audited and state-of-the-art cGMP
facility
• Multi-compendial specification complies with
Ph. Eur., USP-NF, JP, ChP
• Chinese DMF available
Packaging
Packaging
1kg (HDPE container) with PE inner liner
20kg (HDPE drum) with PE inner liner
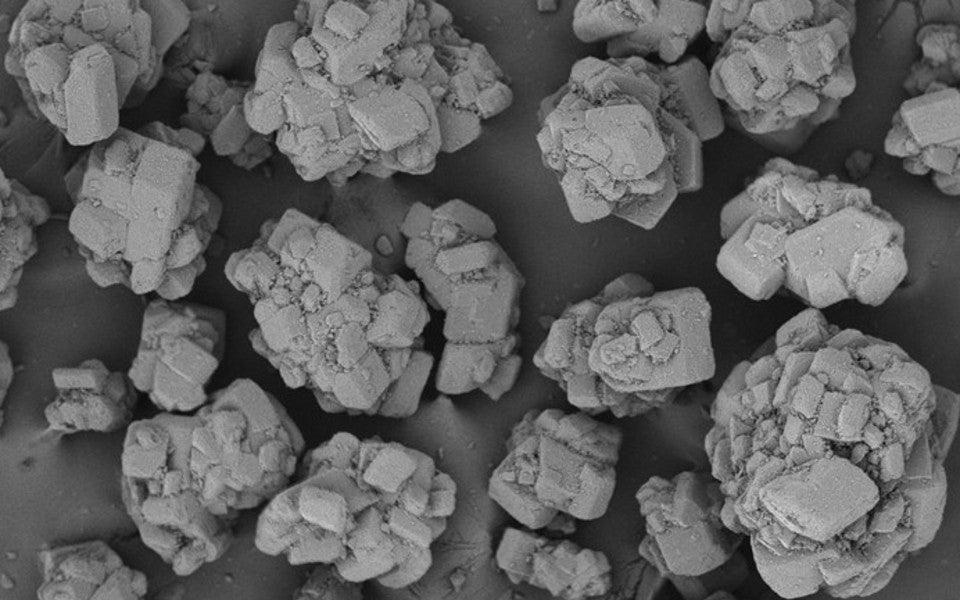


Expected delivery within 5-7 days. Each order ships with Certificate of Analysis, Material Safety Data Sheet and Product Data Sheet.
Let us help you optimize your formulations.
DFE Pharma’s team of technical and regulatory experts, regional sales, and customer service staff is committed to providing unwavering support and reliable guidance at every step of your formulation development and commercialization journey.



