Primojel®
Primojel®
Sodium Starch Glycolate
In 1965, DFE Pharma introduced Primojel® — the world’s first pharmaceutical-gradesodium starch glycolate. Sixty years later, it remains the benchmark in disintegration. Features phosphorous crosslinks — a differentiator that gives it superior chemical stability compared to other SSG brands.
Couldn't load pickup availability
Low stock
Product description
Product description
Primojel® Sodium starch glycolate is a superdisintegrant suitable for a variety of tablet and capsule formulations. In higher concentrations, Primojel® can act as a dissolution enhancing agent. Primojel® is highly effective when used intragranular and/or extragranular in granular formulations.
Benefits
Benefits
Disintegration performance
Primojel® takes up more than 20 times its own weight of water. Rapid water penetration into the tablets and powerful swelling results in rapid disintegration. The swelling actions makes Primojel® most effective in non-soluble matrices. Studies show that Primojel® takes up more water than comparative products on the market and develops a strong disintegrating force making it a highly effective product. The botanic source, degree of cross-linking and degree of substitution of Primojel® have been optimized in order to give rapid water uptake by the polymer without the formation of a viscous gel that may impede water penetration into the tablet.
In direct compression
The recommended starting point is 2%w/w to 4%w/w of Primojel®. The figure shows how the disintegration time of various placebo tablets (250 mg / 9 mm tablets containing 4%w/w Primojel® and 0.5%w/w magnesium stearate) is maintained after storage for 6 months at 40˚C / 75% RH in open containers. The disintegration of reference tablets stored for 6 months under ambient laboratory conditions is also shown. This data confirms that Primojel® remains an effective superdisintegrant.
In wet granulation
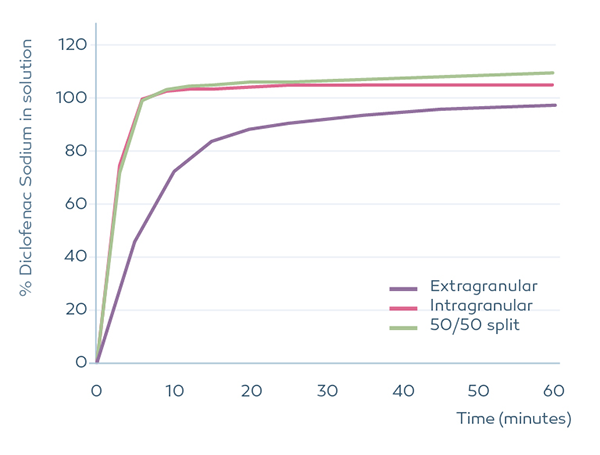
We recommend that Primojel® is incorporated at least partly (50% or more) during the granulation. This is especially important when a high proportion of insoluble diluent is employed. The intragranular components were wet granulated (high shear), dried, sieved and blended with the extragranular portion of Primojel® and magnesium stearate. Tablets were compressed at 250 mg and dissolution tested in 900ml of water using USP apparatus 2. Dissolution of Diclofenac Sodium is slower when Primojel® is used only in the extragranular phase. If lactose is used instead of dibasic calcium phosphate, then the effect of Primojel® location is greatly reduced.
Typical product data
Typical product data
Complies with Ph. Eur., USP-NF, JP
Kosher certified
Particle size distribution
Method: Alpine Airjet
Sieve %w/w <63 µm - min. 93%
Sieve %w/w <125 µm - min. 100%
Source: Potato starch
pH: 6.7
Disintegration mechanism: Swelling and wicking
Product specifications
Product specifications
Hausner ratio: 1,46
Production site: Foxhol, the Netherlands
Quality
Quality
Conforms to USP-NF, Ph.Eur., JP monograph
Packaging
Packaging
Capacity: 50 kg
Shelf life 5 years
Composition: HDPE + PE liner
Sample size: 500g







Let us help you optimize your formulations.
DFE Pharma’s team of technical and regulatory experts, regional sales, and customer service staff is committed to providing unwavering support and reliable guidance at every step of your formulation development and commercialization journey.








