Primellose®
Primellose®
Croscarmellose Sodium
Primellose® is DFE Pharma’s croscarmellose sodium produced in our dedicated pharmaceutical plant in Foxhol, the Netherlands. It’s ideal for tablet and capsule formulations at 2-6% concentration. Primellose® is effective with various filler-binders and works well in both intragranular and extra-granular formulations.
Low stock
Benefits
Benefits
Superior performance
Primellose® is a versatile, robust disintegrant that can be used in wet granulation, dry granulation, and direct compression with nearly all APIs and excipients. Primellose® has strong wicking properties and has superior performance compared to many other brands
Wet granulation
We recommend that Primellose® is incorporated at least partly (50% w/w or more) to get optimal disintegration and dissolution performance in wet granulation formulations.
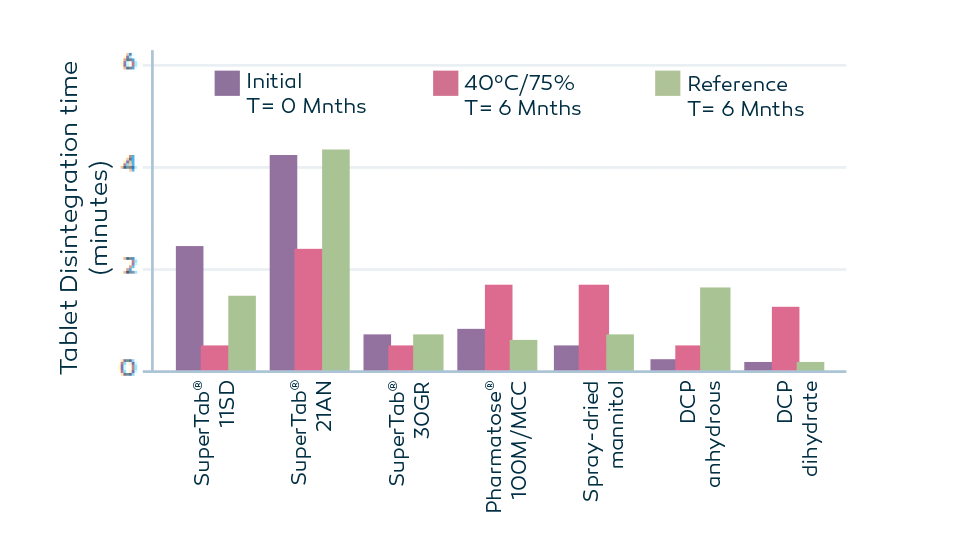
Stable disintegration performance
Primellose® is a robust superdisintegrantt hat retains its performance even under challenging conditions. Testing shows that tablets with 4% w/w Primellose® maintain effective in disintegration even after 6 months of storage at 40°C/75% RH.
Typical product data
Typical product data
Particle size distribution
Sieve %w/w - <45µm - 65-85%
Sieve %w/w - <75µm - min. 90%
Sieve %w/w - <125µm - min. 90%
Quality
Quality
Complies with Ph.Eur., USP-NF, JP
Kosher certified
Packaging
Packaging
- 35Kg drum with PE liner
Shelf life: 5 years
- Sample size: 500g


Let us help you optimize your formulations.
DFE Pharma’s team of technical and regulatory experts, regional sales, and customer service staff is committed to providing unwavering support and reliable guidance at every step of your formulation development and commercialization journey.




