Pharmacel® 101
Pharmacel® 101
Microcrystalline Cellulose 101
Developed specifically as a key component for wet granulation and dry granulation formulations, Pharmacel® 101 Microcrystalline cellulose (MCC) is available from two high quality production sites.
In stock
Benefits
Benefits
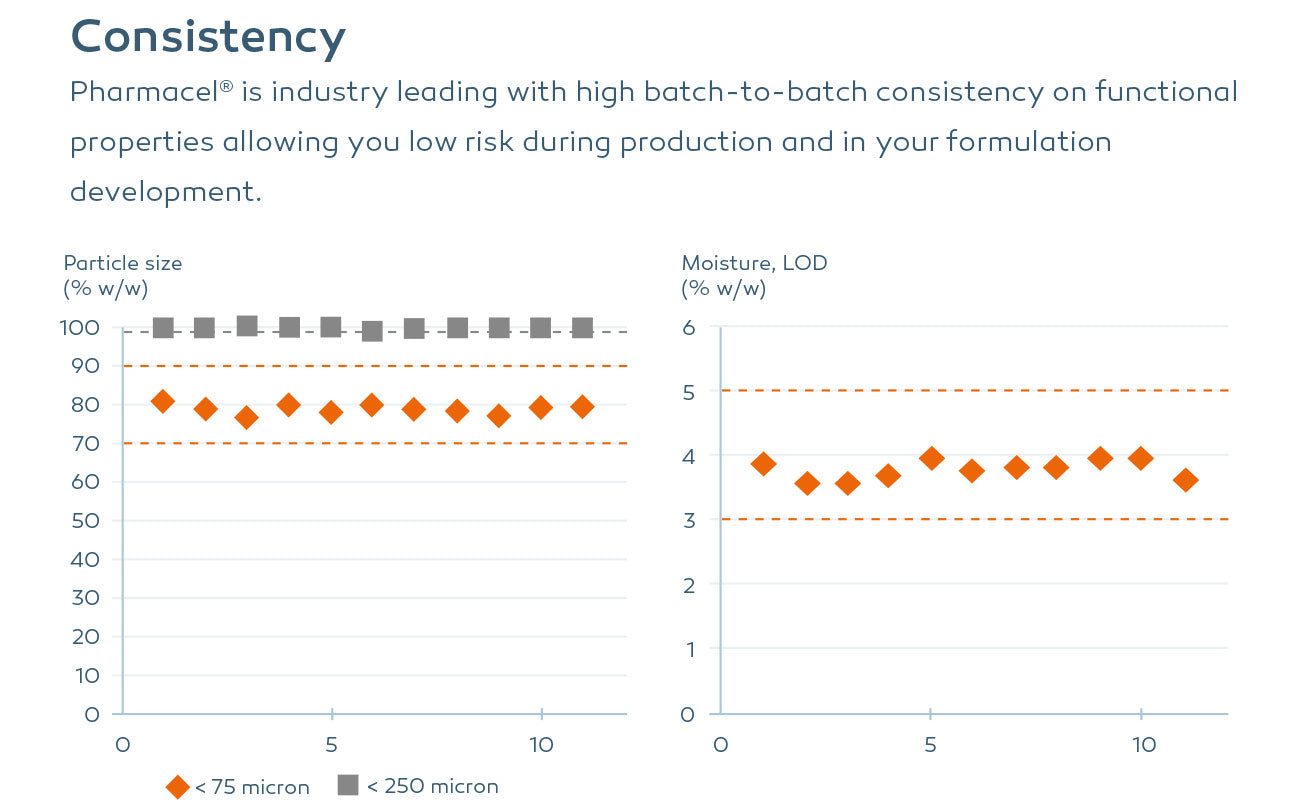
Consistency
Pharmacel® is industry leading with high batch-to-batch consistency on functional properties allowing you low risk during production and in your formulation development. Pharmacel® delivers the highest purity with low nitrites (<0.025 ppm) to de-risk nitrosamines and among the lowest dark particle levels in the industry.
Pharmacel® and lactose formulations
DFE Pharma is the only global excipient manufacturer offering it’s customers both lactose and microcrystalline cellulose products for development of pharmaceutical formulations. Combination of Pharmacel® with one of the lactose grades offered by DFE Pharma provides additional benefit to formulations, by achieving the perfect combination of brittle and plastic excipient behavior.
Wet granulation
Pharmacel® 101 microcrystalline cellulose (MCC) has been developed specifically as a key component for wet granulation formulations. Using Pharmacel® 101 ensures even distribution of the granulating fluid, which in turn means uniform granulation and drying processes. Wet granulation with Pharmacel® 101 is a reliable, easily repeatable and robust process. Tablets made using Pharmacel® 101 show excellent compaction and disintegration properties.
Dry granulation
Pharmacel® 101 microcrystalline cellulose (MCC) is also an ideal excipient for use in dry granulated formulations. Ribbons do not stick to the compaction rolls during roller compaction and dry granulation can be used in formulations with a high drug dosage. For dry granulation Pharmacel® 101 opens up vital production synergies. Used with a brittle excipient, such as SuperTab® 21AN anhydrous lactose, the result is an ideal combination of high tablet strength and insensitivity to re-compaction, leading to outstandingly robust dry granulation formulations.
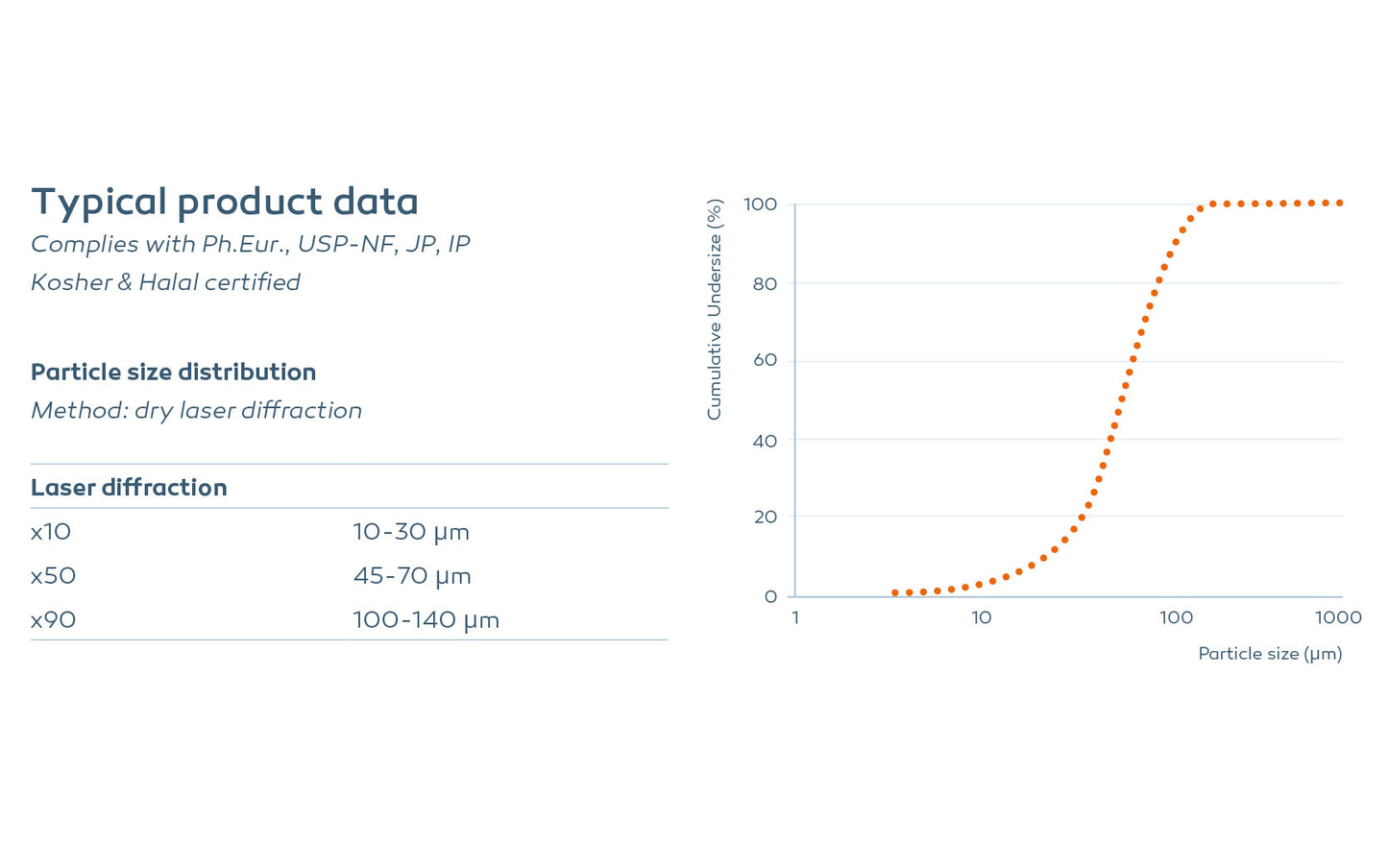
Typical product data
Typical product data
Particle size distribution (μm)
Method: dry laser diffraction
X10: 10-30
X50: 45-70
X90: 100-140
Bulk density (g/L): 280
Hausner ratio (-): 1.57
LOD (% w/w): 3.8
Quality
Quality
Complies with PhEur., USP-NF, JP, IP
Kosher & Halal certified
Packaging
Packaging
Capacity: 25 kg
Composition: Multi-layer PE
Shelf life: 4 years

Let us help you optimize your formulations.
DFE Pharma’s team of technical and regulatory experts, regional sales, and customer service staff is committed to providing unwavering support and reliable guidance at every step of your formulation development and commercialization journey.




